GxP compliance
GxP is the umbrella term for Good “x” Practice guidelines used in regulated industries. The “x” reflects the setting, GMP in manufacturing, GLP in laboratories, GCP in clinical trials and GDP in distribution. Although each discipline has its own rules, the purpose is consistent: quality, safety and reliable records across day-to-day operations.
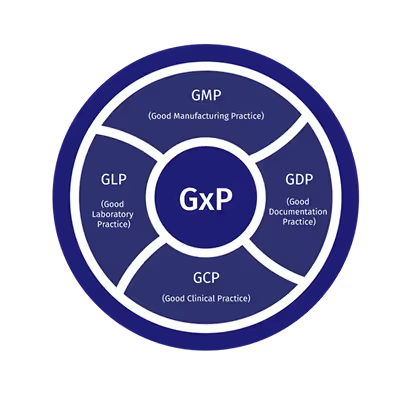
GxP meaning in practice
For organisations in the Healthcare & Pharma, GxP operations cover how products are made and tested, how data is captured and how decisions are documented. The exact obligations depend on your sector and jurisdiction, but common expectations include reliable data, controlled processes and traceable actions.
Core GxP requirements
- Data integrity: complete, accurate, consistent records with access controls
- Traceability & audit trails: who did, what, when and why
- Documented procedures & training: clear SOP's and competent staff
- Qualified/validated systems: equipment and software that perform as intended
Why GxP compliance matter and how digitizing might help
Strong GxP operations help teams avoid production setbacks, reduce rework and pass GxP audits with confidence. They also strengthen trust with regulators, customers and partners, the key to maintaining approvals and long-term relationships. Digital platforms, like RmoniWeb, support GxP by monitoring critical parameters, capturing dependable data, triggering alarms when limits are exceeded and producing audit-ready reports. This makes oversight easier and keeps processes aligned with GxP expectations.

